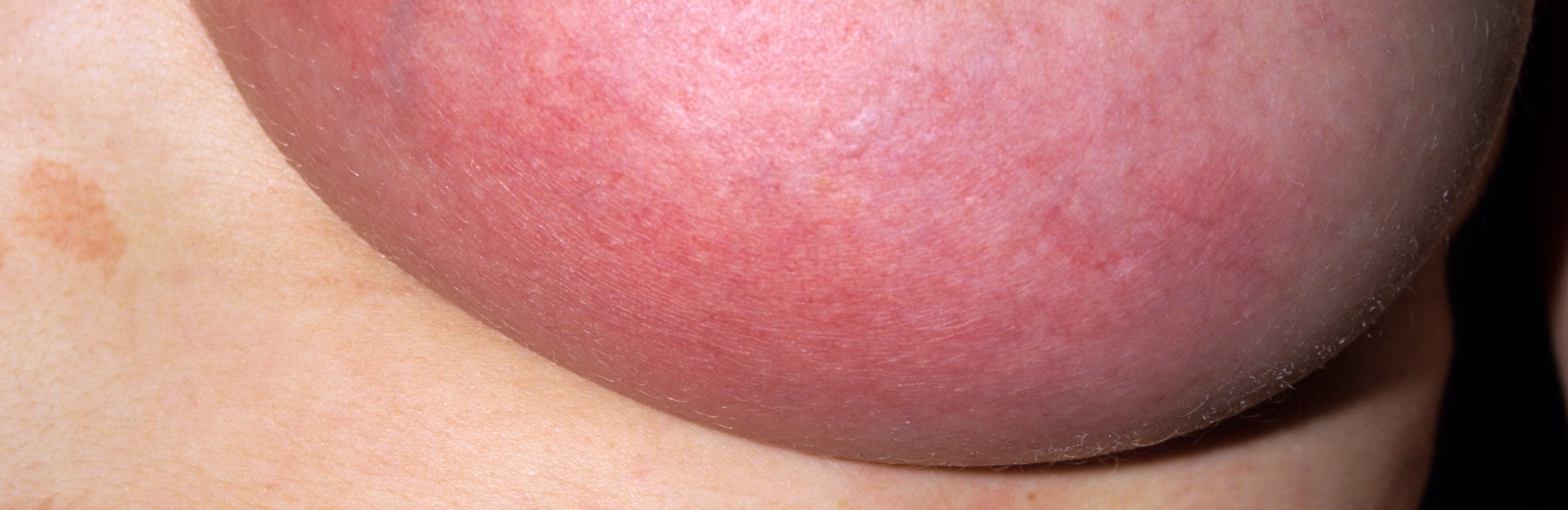
Diagnoses of subacute or subclinical mastitis and mammary dysbiosis don't help breastfeeding patients in the clinic, but promote antibiotic overuse or inappropriate treatments

How is subclinical mastitis and mammary dysbiosis proposed to present?
Subacute mastitis or mammary dysbiosis is characterised as an inflammatory condition which does not present with systemic illness or localized breast erythema.5
Signs and symptoms attributed to subacute mastitis or mammary dysbiosis are detailed in the table below. This table is used in NDC to describe presentations on the spectrum of lactation-related breast inflammation.
| Location of inflammation | Dimensions (millimetres) | Erythema | Pain | Systemtic signs + symptoms |
|---|---|---|---|---|
| Generalised - bilateral | None | None | Feels well | |
| Generalised - unilateral | Mild | Mild when touched only | Fever | |
| Localised WITHOUT lump | Moderate | Mild constant | Myalgia | |
| Localised WITH lump | Severe | Moderate when touched only | Rigor | |
| Moderate constant | ||||
| Severe |
The Academy of Breastfeeding Medicine's Clinical Protocol #36 advises antibiotic treatment for the diagnoses of subacute mastitis and mammary dysbiosis
The Academy of Breastfeeding Medicine's Clinical Protocol #36 advises clinicians that "subacute mastitis occurs when ductal lumens become narrowed by bacterial biofilms in the setting of chronic mammary dysbiosis.” Use of this diagnosis clinically increases the risk of unnecessary antibiotic use.
Because of hypothesised bacterial biofilm formation, ABM recommends two to six weeks of treatment with antibiotics (cephalosporin, amoxicillin/clavulanate, or dicloxacillin, or erythromycin) for diagnoses of subacute or subclinical mastitis or mammary dysbiosis.3, 6-9 Diagnostic criteria are vague, but include nipple white spots. Here are typical treatment protocols:
-
Flucloxacillin 500 mg qid for 2 weeks
-
Cephalexin 500 mg qid if allergic to penicillin
-
Also IV antibiotics.
What mechanisms are proposed to explain the pathogenesis of subclinical mastitis?
-
Proponents of the diagnosis of subacute or subclinical mastitis or mammary dysbiosis have applied the pathogenic microbiota model of breast inflammation to hypothesise that bacterial overgrowth or mammary dysbiosis creates sticky milk or biofilm which causes plugging of ducts, indurated painful areas, decreased milk synthesis, pain with latch, and increased risk of mastitis.2-4 ABM Clinical Protocol #36 hypothesises 1,2 that Candida and coagulase negative Staphylococcus (also Streptococcus, Corynebacterium and Enterococcus) result in dysbiosis. The protocol hypotheses that dysbiosis + biofilm → ductal infection.
-
Some clinicians also hypothesise that women with symptoms of mammary dysbiosis are more likely to develop nipple blebs or white spots, proposing that white spots are distal extensions of biofilm.2, 3, 6 You can read about the pathogenic theory of breast inflammation and why it is inaccurate here.
-
Proponents of the diagnosis of subclinical mastitis also theorise that it is an asymptomatic inflammatory condition of the lactating mammary gland caused by lactocyte tight junction leakage, linked to early lactation failure and poor infant weight gain and which may progress to clinical mastitis. This theory confuses correlation with causation.
In 2018 Kaski and Kvist warned:
"As there is no clear scientific consensus on the definition of human lactational mastitis it would seem incautious to introduce the term 'subacute mastitis': there is certainly no clear definition of what this condition might entail in humans. Also, it would be difficult to prescribe treatment for a condition that has not yet been scientifically described and classified. …. Treatment of a 'subacute' condition should not be recommended as it may be of little value to the individual and might be of great detriment to the global community.” 1
Why NDC guidelines do not use the diagnoses of mammary dysbiosis, subclinical mastitis and subacute mastitis
The NDC mechanobiological model of breast inflammation proposes that there is no role for the diagnoses of subacute mastitis or subclinical mastitis. Mammary dysbiosis is conceptualised as an end-stage overgrowth of a micro-organism, commonly S. aureus, diagnosed by clinical signs of breast inflammation which fails to resolve (over a period of time) with usual conservative measures. In itself, mammary dysbiosis is not a meaningful diagnosis.
-
Using the NDC lens, I propose that underlying unidentified breastfeeding problems may result in asymptomatic inflammatory changes (due to mechanical backpressures), which are measurable in milk by sodium:potassium ratios, immune cell increases, and protein counts. Unless underlying breastfeeding problems are resolved, these changes may in time result in lactation failure and/or poor infant weight gain. From the NDC perspective, the lactating breast is a pro-inflammatory environment, which is excerbated in the context of breastfeeding problems, and a spectrum of changes would be expected prior to clinical presentation. Labelling these asymptomic biochemical and cellular changes with a diagnosis, and proposing clinical interventions (e.g. antibiotic treatment as recommended in ABM Clinical Protocol #36) promotes overtreatments.
-
From the perspective of the mechanobiological model, the previously detailed principles of management of breast inflammation, in particular frequent flexible milk removal and elimination of conflicting intra-oral vectors of force when the infant is suckling, should be applied when there are signs and symptoms of breast inflammation. These two strategies would also be expected to help prevent clinical presentations of breast inflammation.

References
- Castro-Navarro I, Pace RM, Williams JE. Immunological composition of human milk before and during subclinical and clinical mastitis. Frontiers in Immunology. 2025;15:1532432. doi: 1532410.1533389/fimmu.1532024.1532432.
- Ito M, Tanaka M, Date M. Immunological factors and macronutrient content in human milk from women with subclinical mastitis. Journal of Human Lactation. 2024;41(1):26-33 DOI: 10.1177/08903344241297585.
- Kaski K, Kvist LJ. Deep breast pain during lactaton: a case-control study in Sweden investigating the role of Candida albicans. International Breastfeeding Journal. 2018;13:21.
- Betts RC, Johnson HM, Eglash A, Michell KB. It's not yeast: retrospective cohort study of lactating women with persistent nipple and breast pain. Breastfeeding Medicine. 2021;16(4):318-324.
- Mitchell K, Eglash A, Bamberger E. Mammary dysbiosis and nipple blebs treated with intravenous daptomycin and dalbavancin. Journal of Human Lactation. 2020;36(2):365-368.
- Rodriguez JM, Fernandez L. Infectious mastitis during lactation: a mammary dysbiosis model. In: McGuire M, Bode L, editors. Prebiotics and probiotics in human milk: Academic Press; 2017. p. 401-428.
- Samuel TM, De Castro CA, Dubascoux S, Affolder M. Subclinical mastitis in a European muticenter cohort: prevalence, impact on human milk (HM) composition, and association with infant HM intake and growth. Nutrients. 2020;105:doi:10.3390/nu12010105.
- Mitchell K, Johnson HM. Breast pathology that contributes to dysfunction of human lactation: a spotlight on nipple blebs. Journal of Mammary Gland Biology. 2020:http://doi.org/10.1007/s10911-10020-09450-10917.
- Berens P, Eglash A, Malloy M, Steube AM. Persistent pain with breastfeeding: ABM clinical protocol #26. Breastfeeding Medicine. 2016;11:46-56.
- Amir LH, The Academy of Breastfeeding Medicine Protocol Committee. ABM Clinical Protocol #4: Mastitis, Revised March 2014. Breastfeeding Medicine. 2014;9(5):239-243.
- Jimenez E, Arroyo R, Cardenas N, Marin M, Serrano P, Fernandez L, et al. Mammary candidiasis: a medical condition without scientific evidence? PLoS One. 2017;12(7):e0181071.
- Douglas PS. Re-thinking lactation-related nipple pain and damage. Women's Health. 2021:DOI: 10.1177/17455057221087865.
- Douglas PS. Overdiagnosis and overtreatment of nipple and breast candidiasis: a review of the relationship between the diagnosis of mammary candidiasis and Candida albicans in breastfeeding women. Women's Health. 2021;17:DOI: 10.1177/17455065211031480.
- Douglas PS. Re-thinking benign inflammation of the lactating breast: a mechanobiological model. Women's Health. 2022;18:https://doi.org/10.1177/17455065221075907.
- Hooks KB, O'Malley MA. Dysbiosis and its discontents. mBio. 2017;8(5):e01492-01417.
- Brussow H. Problems with the concept of gut microbiota dysbiosis. Microbial Biotechnology. 2020;13(2):423-434.
- Fernandez L, Pannaraj PS, Rautava S, Rodriguez JM. The microbiota of the human mammary ecosystem. Frontiers in cellular and infection microbiology. 2020;10:Article 5866667.